It's very possible that you're not familiar with or have never heard of the term biofilm. But surely, as strange as it may sound to you, you've encountered or come into contact with it in your life! How?
The plaque that forms on your teeth and causes tooth decay is just one type of biofilm. You may have even slipped on rocks coated in it while walking along a stream or river!
When a wound continues to bother you and persists, despite the meticulous care and attention you give it, it is logical to wonder what could be causing this complication... The answer often lies in the presence of biofilm, which delays the healing of your wounds!
But What Is Biofilm and Why Is It Dangerous for Your Wounds?
Bacterial biofilms are colonies of bacteria that often adhere to a surface and to each other, while at the same time being embedded in a self-produced matrix, the so-called biofilm matrix. These colonies are created by many pathogenic microorganisms and their goal is to increase protection from external factors. At the same time, while this matrix provides protection from the environment, the bacteria in turn use various survival strategies to avoid the host's defense systems. In this way, they manage to bypass mechanisms of our immune system and remain on the surface of wounds even more resistant ! It is therefore understandable how their “ power ” is increasing, constantly contaminating your wound.
Recent research has revealed that these bacterial communities exhibit properties, behaviors, and survival strategies that far exceed their capabilities as individual bacteria. For example, bacteria that have formed a colony are naturally tolerant to doses of antibiotics up to 1,000 times greater than doses that kill planktonic bacteria.
Therefore, according to the above, the complex structure of the biofilm and the different characteristics of the microorganisms from which it is created explain their high resistance to various bactericidal substances, including antibiotic resistance . Through the exchange of information between different species, bacteria teach each other to become resistant to antibiotics. As a result, the biofilm becomes impenetrable to antibiotics (topical or oral), common antiseptics or other preparations. At the same time, it reduces chronic inflammatory responses aimed at removing the microbial load.


An article published in Science Direct states that biofilm is involved in the pathogenesis of chronic diseases, especially infections associated with the use of catheters, drains and implant placement. It is also a serious problem in cases of nosocomial infections.
The result of this entire process becomes quite unpleasant and painful for the patient, since he continues to be burdened by the continuous intake of antibiotics without effect while at the same time the healing of the wound is prolonged more and more!
Biofilm Formation as a Factor for Microbial Growth
According to the Center for Biofilm Engineering , bacteria are held together by sugary molecular strands called “extracellular polymeric substances,” or “EPS.” Cells produce EPS and are held together by these strands, allowing them to grow complex three-dimensional communities, or colonies, as discussed above.
How exactly is biofilm created?
In general, wherever there is a combination of moisture, nutrients and a surface, the presence of biofilm is likely . If we think about it, an open wound contains all three of these elements, so the environment is particularly friendly for its growth!
Its development cycle includes the following four (4) stages:
- initial adhesion of microbes to a surface or to each other,
- microcolony formation,
- biofilm maturation and finally,
- dispersion.
Essentially, planktonic bacteria adhere to the wound surface, creating irreversible bonds. In two to four hours, a sticky coating is secreted that becomes increasingly resistant to antibiotics and antiseptics within 6-12 hours. It develops into a mature biofilm in 2 to 3 days and then releases bacteria into the environment which settle elsewhere (stage 4). If it is destroyed mechanically or by the use of antiseptics that cannot penetrate and remove the bacterial communities from the wound , they will re-establish themselves within 24 hours. Thus, they will return again, with full force before the next dressing change.
But what is the solution?
The compact structure of biofilm is what makes it particularly persistent and difficult to eliminate. Bbraun's Prontosan product line was created with the aim of removing and preventing the accumulation of microbial matter on the wound surface.
Prontosan Irrigation Solution, a liquid wound irrigation solution for the prevention of MD class III infections, contains a unique combination of two ingredients, betaine , which is a cleansing surfactant, and polyhexanide , a preservative that allows you to use Prontosan for up to 8 weeks after first opening, preventing infections.
* According to the EU Medical Device Classification (EU MDR) , Prontosan belongs to class III, the highest on the scale. The product is indicated for high-risk cases.
Basic Principles of Wound Biofilm Management
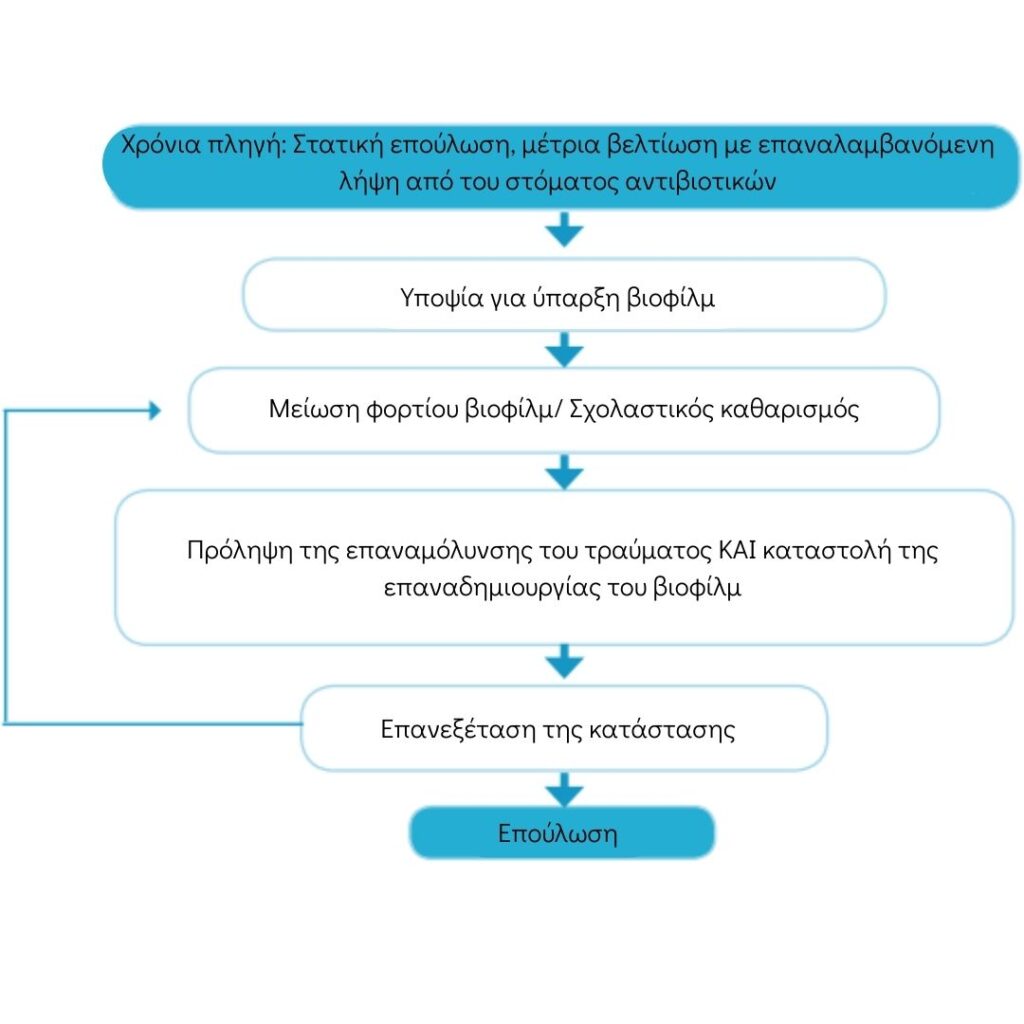
Source : World Union of Wound Healing Societies
The action of Polyhexanide and Betaine on Chronic Wounds
In-vitro experiments have shown that Prontosan liquid wound irrigation solution with 0.1% polyhexanide and 0.1% betaine is the only one in its category that removes, breaks down biofilm and prevents its re-formation! Research has shown that wound size was significantly reduced in just 2 weeks of use.
Betaine lifts and removes debris and microbial load from the wound, while polyhexanide is a polymer with antiseptic properties against a wide range of microorganisms.
Tip! For best cleaning results, rinse, soak and re-irrigate the wound with Prontosan Wound Irrigation Solution . Applying Prontosan Wound Gel X extends the action for up to 72 hours, or until the next dressing change. Biofilm cannot be removed with a single application. Repeated applications are required for optimal results.